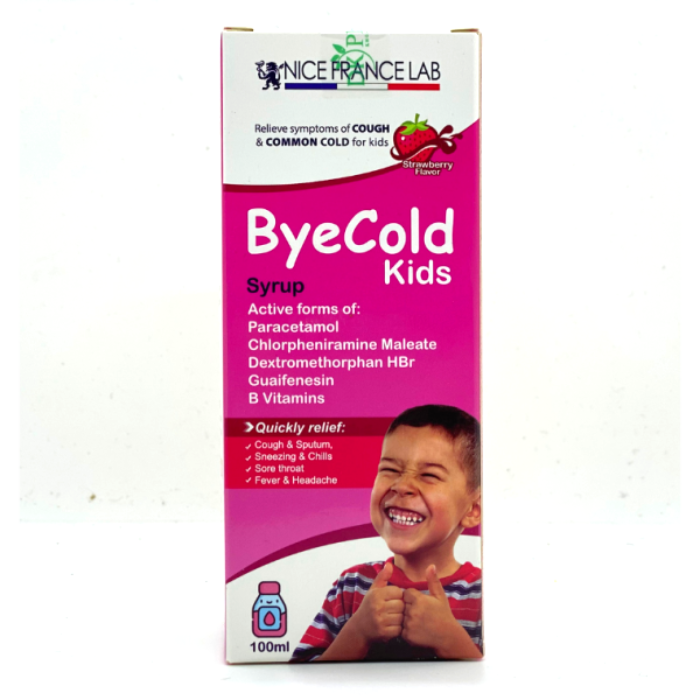
1. Dosage form:
Syrup
2. Packing:
Box of 01 bottle of 100ml
3. Compotion:
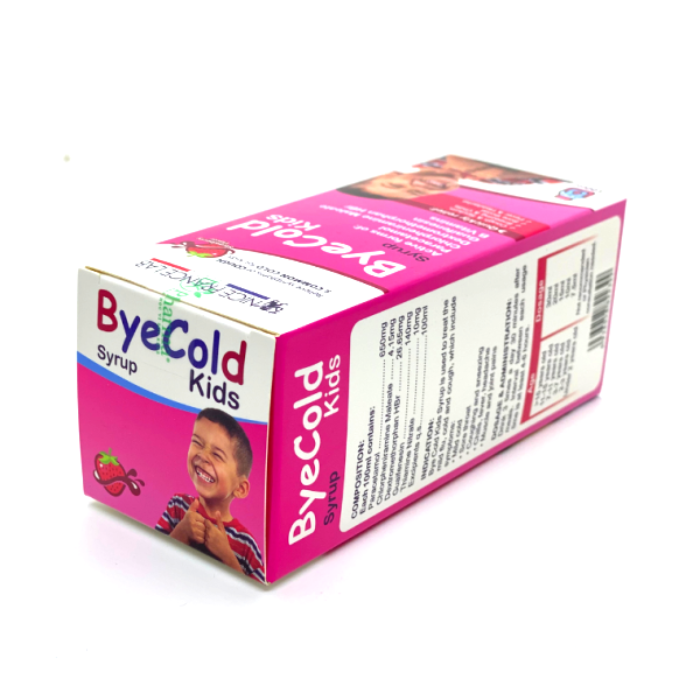
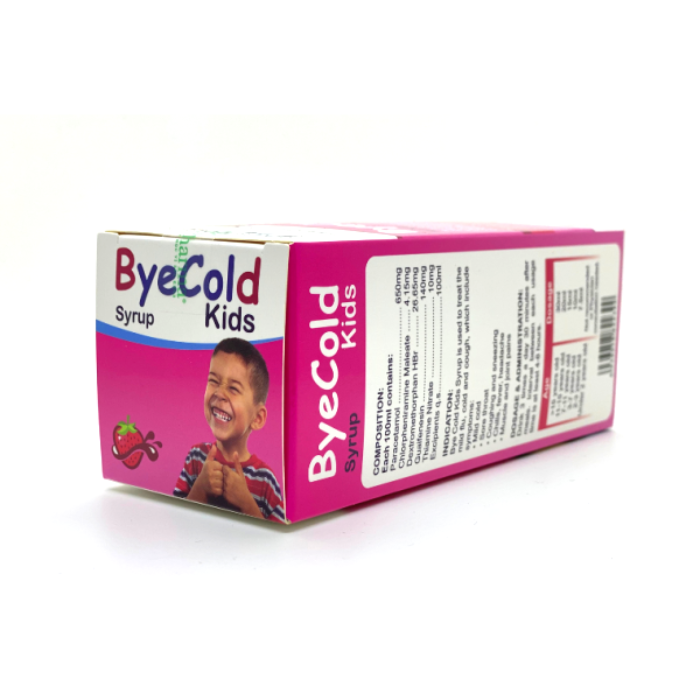
Each bottle of 100ml contains:
Paracetamol ……………………………………… 650mg
Chlorpheniramine Maleate …………………. 4.15mg
Dextromethorphan HBr ……………………. 26.65mg
Guaifenesin ………………………………………..140mg
Thiamine Nitrate ………………….10mg
Excipients q.s……100mI
4. Indication:
Bye Cold Kids Syrup is used to treat the mild flu, cold and cough, which include symptoms:
- Mild cold
- Sore throat
- Coughing and sneezing
- Chills, fever, headache,
- Muscle and joint pains
- Bye Cold Kids Syrup have sweet tasty strawberry flavor in oral syrup dosage form, which is very convenient and easy for kids to use,
5. Dosage & Administration:
Intervals at least 4-6 hours between each dose:
- 15 years and older: 30ml
- 11 —15 years old: 20ml
- 7 — 11 years old: 15ml
- 3 — 7 years old: 10ml
- 2 — 3 years old: 7.5ml
6. Notice:
Kids under 2 year-old is not recommended for use or need to be consulted with physician carefully before use.
- Do not use product continuously in 7 days without the consultation of doctor or physician
- Do not use for self-treatment of paint or fever longer than 10 days in adult and 5 days or high fever ( 39.5 ° C) lasting more than 3 days in children unless indication from doctor or medical health care because long lasting pain and fever can be symptoms of other disease that need serious diagnose .
7. Contradication:
- Patient hyper sensitive to ingredients of drug.
- Patients with severe liver failure, glaucoma, urinary retention due to urethral disorder, prostate, anemia or have heart disease, lung, kidney.
- Patients with glucose – 6 – phosphate dehydrogenase deficiency disease.
- Breastfeeding, infants and premature babies.
8. Precaution:
- Paracetamol is relatively non-toxic with therapeutic doses. However to urinary phenylceton patient or person who have to limit the amount of phenylalanine intake, should avoid use paracetamol with drug or food containing aspartame. Caution in use at patient having prehistory of anemia, liver and kidney failure. Intake of alcohol drinks or wine can increase the toxic of paracetamol to liver, therefore patient should avoid alcohol and wine during use of product.
- Chlorpheniramine maleate can increase the potency of urinary retention, especially in especially in people with prostatic hypertrophy, obstruction of the urinary tract, duodenal stricture, and aggravated in the muscular dystrophy. Sedate effect of chlorpheniramine is increased when use together with ancohol or other sedative product. Caution in use with patient of chronic lung disease, shortness of breath, people with glaucoma, the elderly. The risk of tooth decay when used for a long time.
- Patient use product containing paracetamol should be warning about signs of severe skin reactions such as Stevens-Johnson syndrome
(SJS), toxic necrotic skin syndrome (TEN) or Lyell’s syndrome, Acute Rhinitis (AGEP).
9. Dextromethorphan HBr:
- Avoid simultaneous use with nonselective monoamine oxidase inhibitors (MAO inhibitors) – serotonin syndrome
- Contains aspartame, use caution in patients with phenylketonuria
- May cause hallucinations, confusion, agitation, overactive reflexes, shivering, twitching of muscles and rapid heart rate
- Not for use in children under 4 years old and use caution when using in children younger than 6 years old
- Use caution in patients who are sedated, debilitated or confined to a supine position.
10. Guaifenesin:
- Guaifenesin may alter some laboratory tests. It may increase renal clearance for urate and lower serum uric acid levels. Guaifenesin may produce an increase in urinary 5-hydroxyindoleacetic acid and may therefore interfere with the interpretation of this diagnostic test for carcinoid syndrome. Guaifenesin may also falsely elevate the VMA test for catechols. Guaifenesin should be discontinued at least 48 hours prior to the collection of urine specimens for such laboratory tests. Guaifenesin should not be used for persistent or chronic cough such as occurs with asthma, emphysema, or chronic bronchitis or any other condition where cough is associated with excessive secretions, unless under the supervision of a health care professional. Guaifenesin should not be used for a cough that is specifically associated with heart failure or ACE inhibitor therapy.
- A fever may be indicative of a serious condition. Guaifenesin should be used in patients with a high temperature only under the direction of a physician.
- Guaifenesin products are not recommended for use in children and infants less than 2 years of age due to lack of evidence for safety and efficacy.
- Keep out of reach of children. Consult your physician carefully before use. In case of overdose or ADRs happens, get medical help or contact a Poison Control Center immediately
11. Use in pregnancy & lactation women:
- Pregnancy woman: only use in necessary situation. Use Chlorpheniramine maleat in the last 3 months of pregnancy can cause serious
reaction (seizures) in infant. - Lactation woman: use with careful consideration: not use product or not breast feeding during use of product, depend of the necessity of
drug to mother. - Consult doctor or physician carefully before use.
12. Drug interaction:
Intake of paracetamol in long time and high dose slightly increase the anticoagulant effect of coumarin and indandion derivates. Use product together with phenothiazine and heat-reduction therapy can lead to serious over heat-reduction consequence. Anticonvulsants (phenytoin, barbiturate, carbamazepine), isoniazid, and anti-tuberculin drugs may increase the toxicity to the paracetamol to liver. Excessive and long-term drinking may increase the risk of paracetamol poisoning the liver. Cholestyramine reduces paracetamol
absorption (not drinking within 1 hour).
Dextromethorphan HBr has severe interactions with:
- Isocarboxazid
- Procarbazine
- Safinamide
- Tranylcypromine
- Phenelzine
- Rasagiline
- Selegiline
Dextromethorphan HBr also has interactions with other drugs at different level of severity, consult your doctor carefully before use this product at the same time with other drugs.
13. Adverse drug interaction:
Relate to paracetamol:
Common 1/1000<ADR<100
Skin: skin rash. Gastrointestinal: nausea, vomiting. Hematology: hematopoietic disorders (neutropenia, reduction of whole blood cells,
neutropenia), anemia. Renal: Kidney disease, kidney toxicity in long-term abuse.
Rarely, ADR<I/1000
Skin: Stevens-Johnson syndrome, toxic epidermal necrolysis, Lyell’s syndrome, acute onset epiglottis. Other: hypersensitivity reaction.
Relate to Chlorpheniramine:
Unwanted effects are reported at frequencies (1 to 10%), very common (2 10%). The incidence of other side effects after delivery to the market has not been documented.
Very common:
Central nervous system: drowsiness, sleepiness.
Frequent:
CNS: attention deficit disorder, dizziness, headache. Gastrointestinal: Nausea, dry mouth. Vision: blurred vision. Other: tired.
Unknown frequency:
Blood and lymphatic system: hemoptysis, hemolytic anemia. Immune system: allergic, angioedema, sensitive shock. Metabolism and nutrition: anorexia. Mental illness: dizziness, irritability, irritability, nightmares, depression. For hearing and inner ear: tinnitus. Cardiovascu- lar system: tachycardia, arrhythmias. Blood vessels: low blood pressure. Respiratory, thoracic, mediastinal: increased bronchial secretion.
Gastrointestinal disorders: vomiting, abdominal pain, diarrhea, dyspepsia. Liver: hepatitis, jaundice. Skin and subcutaneous tissue:
dermatitis, rash, urticaria, sensitive light. Musculoskeletal and connective tissue: muscle twitching, muscle weakness. Renal and urinary:
Urinary retention. Other: chest pain
14. Related to Dextromethorphan HBr:
Slight drowsiness/dizziness, nausea, or vomiting may occur. Rarely, some people may experience severe drowsiness/dizziness with normal
doses. If any of these effects persist or worsen, tell your doctor or pharmacist promptly.
If your doctor has directed you to use this medication, remember that he or she has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
A very serious allergic reaction to this drug is rare. However, seek immediate medical attention if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
Caution: Children and adults with undesirable side effects are more likely to be associated with cholinergic resistance and back stimulation
(eg restlessness, anxiety). Inform your doctor if any of the side effects occur when using the medicine.
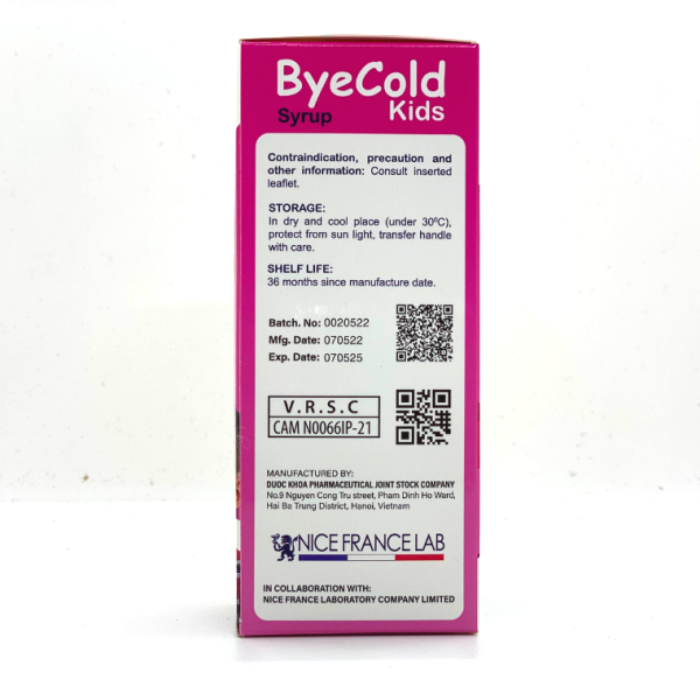
15. Storage:
Dry and cool place, below 30 ° C, protect from light and moisture.
16. Shelf life:
36 months since manufacture date.
17. Standard:
In house.